Get the latest tech news
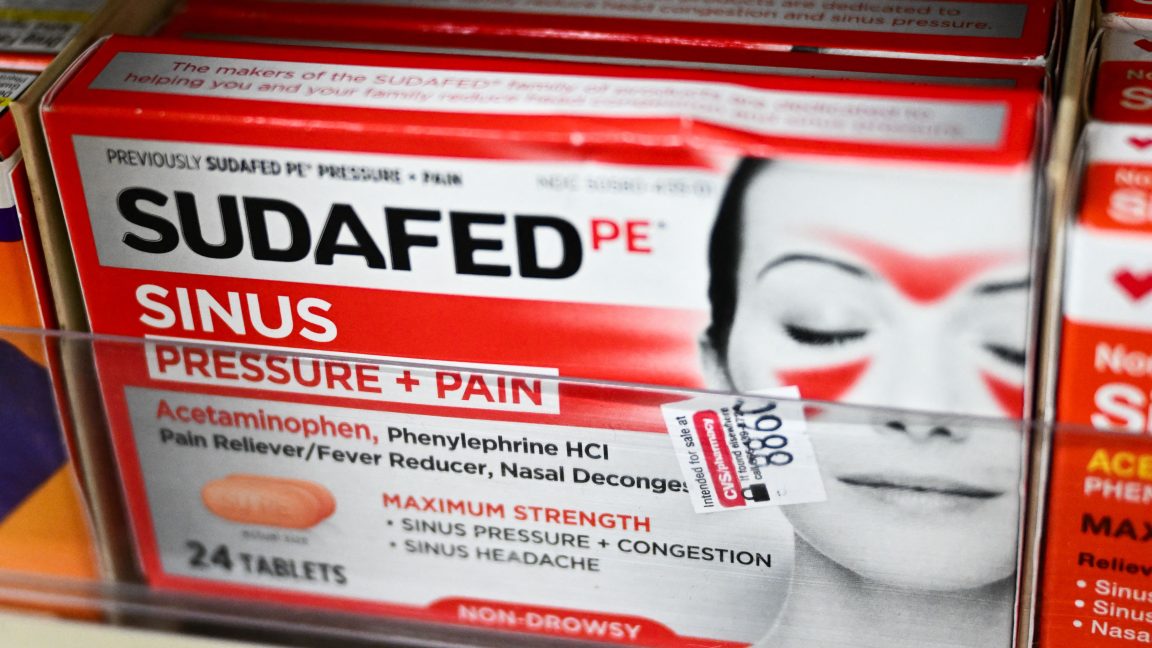
FDA proposes ending use of oral phenylephrine as OTC nasal decongestant
FDA is proposing to end the use of oral phenylephrine for the temporary relief of nasal congestion because the agency determined it is not effective.
“Based on our review of available data, and consistent with the advice of the advisory committee, we are taking this next step in the process to propose removing oral phenylephrine because it is not effective as a nasal decongestant.” Last fall, the FDA also held a Nonprescription Drug Advisory Committee meeting to discuss the ‘Generally Recognized as Safe and Effective’ (GRASE) status of oral phenylephrine as a nasal decongestant. The committee discussed new data on the effectiveness of orally administered phenylephrine and unanimously concluded that the current scientific data do not support that the recommended dosage in the OTC cold, cough, allergy, bronchodilator and antiasthmatic drug products monograph for orally administered phenylephrine’s effectiveness as a nasal decongestant.
Or read this on Hacker News